How oxygen is used in cutting?
Oxygen cutting is based on the chemical reaction between oxygen and metal at high temperatures. The necessary high temperature is obtained from an oxyfuel gas flame, the most popular fuel gas being acetylene.
The process is therefore commonly referred to as the oxyacetylene process and is widely used in all metal fabrication shops. A wide range of oxy-cutting machines are available, with a simple manual cutting torch at one end and the most sophisticated design with an electronic tracking device at the other end.
Stage for Oxy-Fuel Cutting (OFC)
Oxy-fuel gas cutting is a thermal cutting process. According to the degree of mechanization, we can distinguish between hand cutting (manual cutting), partly mechanical, fully-mechanized, and automated cutting.
The fundamental principle for oxyfuel gas cutting is in two stages:
- Convert the metal into its oxides ( material formed with the reaction with oxygen) so that it can no more able to join once it’s solidified.
- Using high-pressure oxygen gas, removes these metal oxides to form the gas cut.
This process works best for materials where they can easily form metal oxides such as Iron (Fe) forms iron oxide when it is heated in the presence of oxygen. The higher the heating temperature, the more the number of oxides formed.
During the torch cutting, the material is burned by the heat of the flame and these combustion products (metal oxides) are expelled by an oxygen jet of high kinetic density.
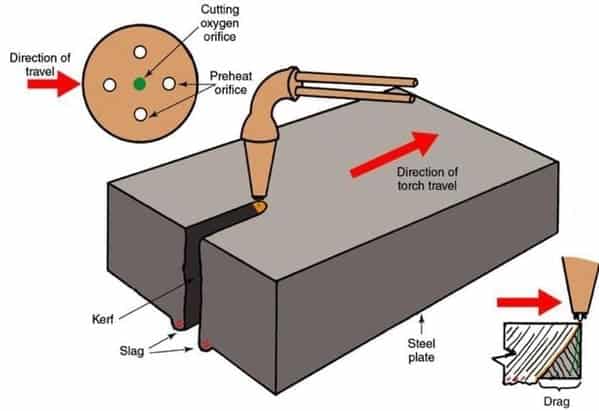
Process principle of flame cutting
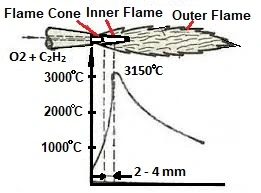
- The heating of the workpiece is to be cut by the heating flame to ignition temperature in the area of the effective zone of the cutting oxygen jet. The ignition temperature depends on the carbon content of the steel at 1,150 °C to 1,200 °C.
- Supply of oxygen and thus the introduction of the combustion of the material inside of the kerf, whereby the exothermic reaction of the material with oxygen allows considerable amounts of heat to be released.
- Exhausting the burned material (the slag) from the kerf by means of the cutting oxygen jet.
- The kerf is generated by the uniform movement above the workpiece.
Why is Stainless or Aluminum can not be cut by oxyfuel torch cutting?
Stainless steel and aluminum have an oxide layer on their outer surface and the melting temperature of these oxides is much higher than the base metals.
For example, the melting point of aluminum is 660 Degree Celsius (1220°F) but the melting point of its oxide is 2050 Degree Celsius ( 3722°F).
If we try to cut it with oxyfuel gas cutting, the oxygen jet or flame can not reach the metal as it is covered by a solid oxide layer as shown in the below picture.
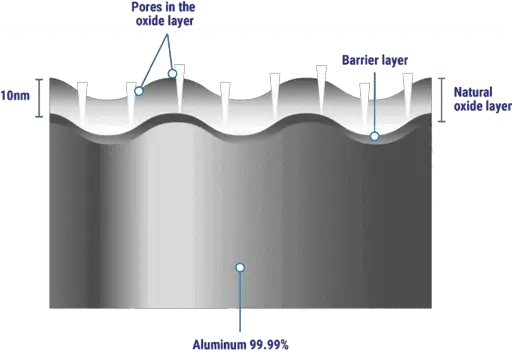
Due to this reason, aluminum materials are not suitable for gas cutting. The other reason is the high thermal conductivity of the aluminum material.
A similar case is for stainless steel or material having high chromium as chromium forms a chromium oxide layer when it came to contact with oxygen in the atmosphere.
The melting point of Stainless steel is around 1400-1450°C (2552-2642°F) while the melting point of chromium oxide is 2435°C (4415°F).
So, the flame will not be able to cut through the oxide layer as shown in the below picture.
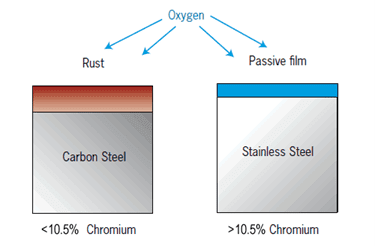
Before we will melt the oxide layer, the base metal will get melted and we will not be able to cut the material and it will only result in a shabby look melted area.
More the alloying elements in the steel, more the difficult to cut the material with flame or torch cutting using oxyfuel gases.
Aluminum and its alloys and stainless steels are also difficult to cut, because the oxides of aluminum and chromium have melting points much higher than those of iron oxides.
These high-melting oxides, which are refractory in nature, continuously accumulate in the kerf as cutting proceeds and prevent exposure of fresh iron to the cutting oxygen stream.
Special oxygen-cutting techniques using metal powder or chemical flux are therefore employed for cutting these difficult metals.
For getting clean and efficient cuts at high speeds in these metals, arc-cutting processes are preferred.
Why is cast iron flame cutting difficult?
It is difficult to cut cast iron by the normal oxyfuel process, because of the presence of graphite and iron carbide which hinder the oxidation of the iron that is pre-requisites to start the cut.
Due to these elements, flame cutting or oxy fuel cutting or oxygen cutting processes can not be used for cutting of cast iron.
Oxyfuel cutting of Cast Iron
The cutting of cast iron is also not possible with oxyfuel gas cutting. Here, cast iron means a material having more than 2% Carbon.
The reason is the presence of graphite in the microstructure. The melting temperature of graphite is 3,600°C while the cast iron melting temperature is 1300°C (vary based on carbon percentage).
Many people confuse cast iron with cast steel. Cast steel can be easily cut with oxyfuel gas cutting as it is just plain carbon steel but in casting form.
Due to this reason, cast steel is easy to cut with gas cutting. Some cast iron, microstructure does not contain graphite and hence they are easy to cut with gas cutting or let us oxyfuel gas cutting or torch cutting.
How to cut stainless steel plate/ pipe material?
The best and most effective method to cut stainless steel or aluminum is by Plasma cutting or laser cutting. A small cut can be made using the cutting wheel in the shop.
Water jet cutting is another option that does not produce any thermal effect in the materials. In water jet cutting, the high-pressure stream of water along with abrasive material cut the material.
Easy trick to cut stainless steel in Shop when Plasma or laser cutting is not available.
It is many times we face difficulty in the shop when we don’t have laser cutting or plasma cutting.
The cutting with a cutting wheel is good for small thickness but what if you have to cut thicker parts, let’s say 20 mm thick plate?
In such cases, welding can be used to cut material although it’s used as joining material. What you need to do is take a 3.2 mm (1/8”) electrode (best is a cellulose rod- E6010/ E6011, as they have high arc pressure), set the machine to a high current let’s say 200- 250 ampere. Mark the area to be cut and weld on it.
Due to high current and heat, the material will melt and form a cut. Although, not a perfect cut like a laser, you can easily and fast cut the material. Just do a little grinding and you will be good to go.
TIG and MIG Arc Cutting
The normal TIG welding process can be converted into a cutting process by increasing the current and rate of inert gas flow.
The moderately high velocity of the gas, which is usually a mixture of argon and hydrogen, blows away the molten metal to form a kerf. Using currents in the range of 200-600 amp, stainless steel and aluminum up to 13 mm thick can be cut with ease. The MIG process can also be used for cutting in a similar way.
Considering the expense of inert gas and electrodes, TIG and MIG cutting processes are rarely used in industry. Hence they are not mentioned in the chart.
Today, the most efficient and commonly used processes are plasma arc cutting and air carbon-arc cutting.
Preconditions for flame cutting
To enable the start of the heat-generating (exothermic) process, the material to be cut must meet the following requirements:
- The material heated to ignition temperature must burn in pure oxygen. This requirement is met by all metals with a sufficiently high affinity to oxygen; it is met particularly well by pure iron.
- The slag generated during the combustion must be fluid, so that it can be blown out from the kerf by the oxygen. In particular chrome and silicone form viscous slag.
- Its ignition temperature must be lower than its melting temperature. The ignition temperature of structural steels is approximately 1,200 °C, the melting point is just around 1,500 °C. Such materials thus can burn before they become liquid. With increasing carbon content, the burning temperature increases as well, and the melting temperature decreases. For steels of about 1.6 % carbon content, this requirement is no longer met – the material melts before it is burned. Therefore, e.g. tool steels and cast iron are not suitable for flame cutting.
- The melting temperature of the oxides must be lower than the melting temperature of the material. Some metals and alloying elements form highly melting oxides. A typical example is an aluminum. Its melting point is at 660 °C, the melting point of its oxide at approximately 2,050 °C. The oxygen jet cannot even reach the metal as it is covered by a solid oxide layer. Aluminum materials are therefore not suitable for flame cutting. It is similar with chrome, which also forms highly melting oxides. As nickel only has a low affinity to oxygen, it contributes not much to the combustion heat.
This is the reason, why stainless CrNi steels are not suitable for flame cutting. Also some other alloying elements of steel such as silicon, manganese, tungsten, molybdenum and copper make – in high amounts – flame cutting more difficult. - The emerging oxides need to be thin fluid. If, during combustion, a slag is built that is very viscous and thus cannot be expelled from the kerf easily, flame-cutting can naturally be impeded. This characteristic is also influenced by chrome and silicon.
- The heat conductivity of the material may not be too high. Namely, if more heat is dissipated as it is added during combustion, the cutting process dies down – especially in deeper layers of the material that the heating flame does not reach. This condition is the case with copper, for instance.