Sensitization of Stainless Steel
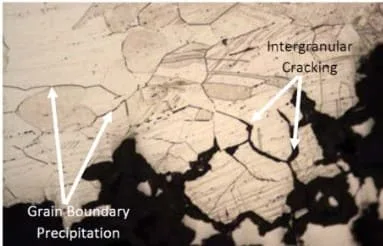
Sensitization of stainless steel occurs when stainless steel is heated to between 1100°F-1560°F (600°C to 850°C). At this temperature range, chromium carbides can form on the grain boundaries of the austenitic stainless steel.
These chromium carbides are hard and brittle, and they can make the steel susceptible to corrosion.
Sensitization can be caused by several factors, including welding, heat treatment, and exposure to high temperature during service.
Sensitization can be prevented by using proper welding techniques and avoiding exposure to high temperature.
how to prevent sensitization of stainless steel?
Sensitization of stainless steel is the formation of chromium carbides at the grain boundaries. When sensitization occurs, the steel becomes susceptible to corrosion. There are several ways to prevent sensitization:
- Use a low carbon content steel. The carbon atoms can combine with the chromium atoms to form carbides, so using a steel with a lower carbon content will reduce the amount of carbides that can form.
- Use a stabilizing element such as niobium or titanium. These elements help to keep the chromium in solution and prevent it from combining with carbon to form carbides.
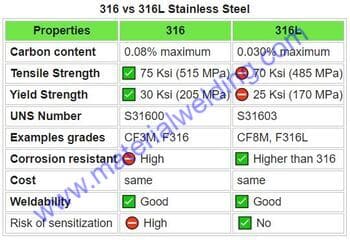
- Use an austenitic stainless steel. This type of steel has a higher percentage of chromium and nickel, which helps to prevent sensitization.
- Avoid welding or heat treatments that can cause sensitization.
This results in a loss of corrosion resistance and can be prevented by using proper heat treatment techniques if sensitization already occurred in the material.
To remove the effect of sensitization, material or welded parts need to be solution annealed.
In solution annealing, parts are heated above the austenization temperature to dissolve all carbides and then cooled rapidly to prevent their reformation.
Sensitization Corrosion
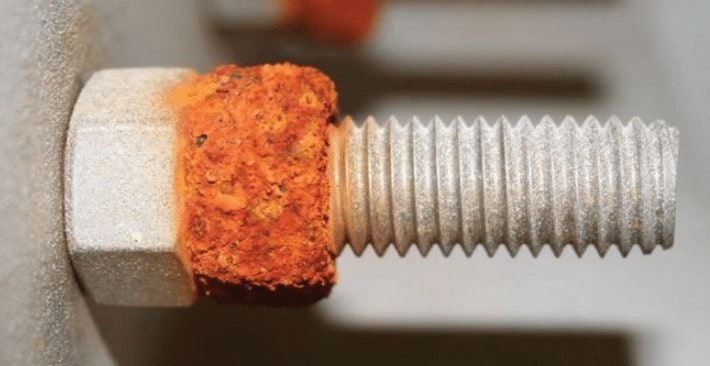
Sensitization corrosion is a type of corrosion that can occur in stainless steel and other alloys. It is caused by the exposure of the metal to high temperatures, which causes the formation of chromium carbides.
These carbides can make the metal more susceptible to corrosion. Sensitization corrosion can be prevented by using alloys that are resistant to it, such as duplex stainless steels.
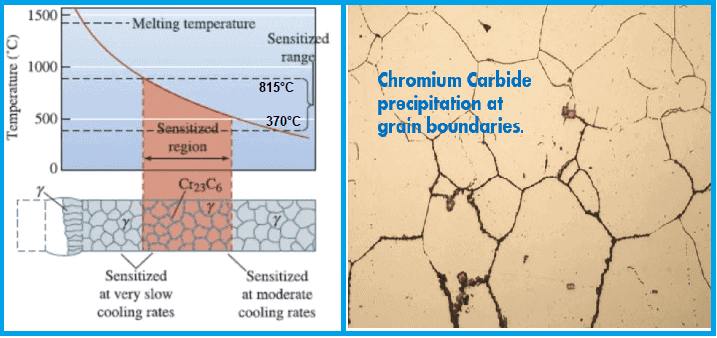
Sensitization Metallurgy
Sensitization is a heat treatment process that alters the microstructure of austenitic stainless steels to make them more susceptible to intergranular corrosion.
The process involves heating the steel to a temperature above its critical temperature and then cooling slowly to allow carbide formation. This produces a microstructure that is rich in chromium carbides.
The carbides are preferentially located at the grain boundaries and act as barriers to the diffusion of chromium atoms. This decreases the ability of the steel to resist intergranular corrosion.
Austenitic stainless steel welding problems
Austenitic stainless steel is a type of stainless steel that contains austenite. This austenitic microstructure is achieved by adding at least 10% chromium to the steel.
Austenitic stainless steels are not hardenable by heat treatment and are therefore generally not required property alteration by heating.
They can be welded, however, and are often used in food processing and chemical handling equipment because of their corrosion resistance.
Austenitic stainless steels can be difficult to weld if not welded with proper technique.
When welding austenitic stainless steel, it is important to use a low-carbon filler metal. This will help to prevent carbon from diffusing into the austenite and causing it to become brittle. Another problem that can occur when welding austenitic stainless steel is hot cracking.
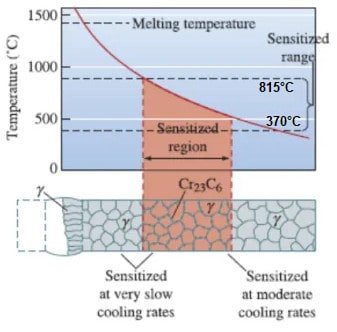
sensitization temperature
Sensitization is the process of heat treating steel to promote carbide precipitation. The temperature range for sensitization is 1100°F-1560°F (600°C to 850°C).
This temperature range allows for the formation of chromium carbides, which are hard and wear-resistant. Sensitization increases the hardness and strength of steel, and makes it more resistant to wear and tear.
Sensitization in welding
Sensitization in welding is a process that involves the exposure of metals to high temperatures. This can cause the metal to become more susceptible to corrosion.
Sensitization can occur during the welding process or after the weld has cooled. When sensitization occurs, it is important to take steps to prevent corrosion from occurring.