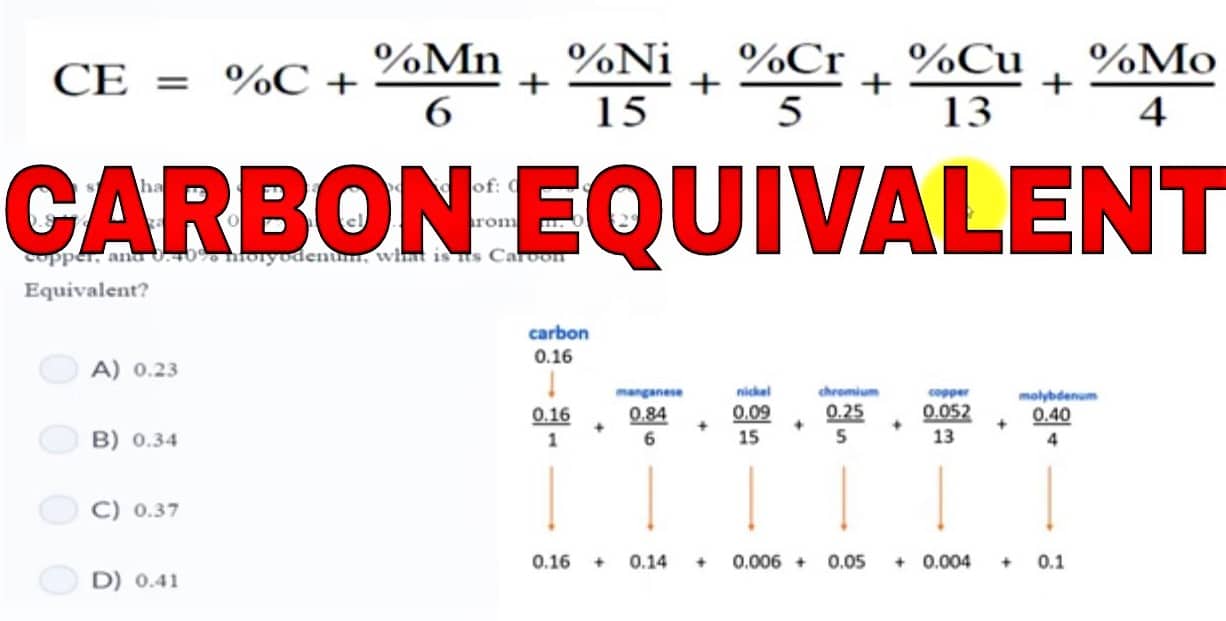
Steel is primarily an alloy of iron and carbon and certain additional elements such as Manganese & silicon. Knowing the Effect of alloying elements in Steel properties is a fascinating topic.
Alloying here refers to the addition of other elements to achieve the desired mechanical (higher tensile, yield, toughness etc.), physical (Hardness, color etc.), and chemical properties (e.g. corrosion resistance).
Effect of Alloying elements in Steel / Iron & Stainless Steel
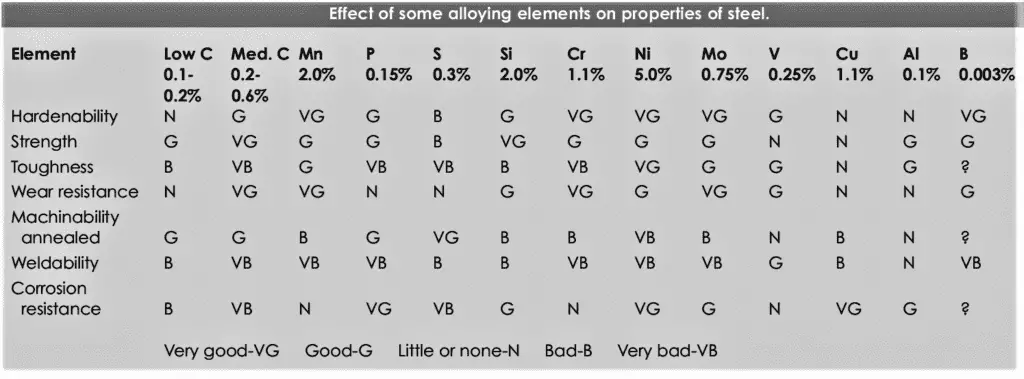
Each alloying element has its own influence on the mechanical/ chemical properties of steel. In this post, you will learn the most of the mainly used alloying elements, their effect on the properties of steel with their addition as well as their importance for a Welding, material, metallurgist, and QA-QC Engineers.
The below table summarizes the effects of alloying elements in steel. For a detailed explanation, continue through the post.
Carbon (C) effect on steel
Carbon is a strong austenitic stabilizer, that increases the tensile strength of steels by increasing the amount of carbide present. Carbon increases the hardening capacity of the steel so that it may be effectively quenched and tempered. Carbon with its unique effects on steel provides allotropic transformation to the steel.
Carbon strongly decreases the toughness & corrosion resistance in ferritic steels. In martensitic steel, carbon gives rise to hardness & strength but reduces material toughness. This effect is more when present as lamellar (layered) cementite in pearlite rather than round (globular/spheroidal) particles.
Silicon (Si) effect on steel :Effect of Alloying elements in Steel
Silicon increase oxidation resistance. This resistance is at high temperature and at lower temperatures. Silicon being a ferrite stabilizer promotes ferritic microstructures. Silicon increases strength in steel along with its primary function as a deoxidizer. It moderate increase in hardening capacity.
Manganese (Mn) effect on steel:Effect of Alloying elements in Steel
Manganese is added up to 1.8 wt%. It combines with sulfur to form less harmful manganese sulfide inclusions in high sulfur steels thus preventing issues of hot cracking during welding. It Increases the steel’s strength but less than silicon. It helps to improve the steel’s toughness to room temperature. Manganese considerably increases the steel’s hardening capacity.
It can be added as a deoxidant during melting or in welding fillers. Its presence will therefore assist in the workability of the metal by the initial manufacturer. Manganese helps to remove the harmful effects of Sulphur through the formation of MnS rather than Ni3S2 and increases the solubility of nitrogen in nickel based alloys.
MnS is important for stainless steels where the MnS particles act as corrosion initiation sites. It must be kept low for high corrosion resistance in these alloys. Manganese also increases the magnetic permeability.
Nickel (Ni):Effect of Alloying elements in Steel
Nickel has a high resistance to carburization and oxidation. Nickel is a austenitic stabilizer promoting Face-Centered-Cubic microstructure. It gives rise to alloy ductility and toughness.
Nickel is beneficial for alloy weldability, thus it is added intentionally to martensitic stainless steel alloys. Due to its strong FCC stabilization effects, Nickel is the main element for cryogenic stainless steel & 9% Nickel steel.
Nickel has Little effect on steel’s strength and hardening capacity but considerably improves its low temperature toughness by promoting stable austenitic even at room temperature. Nickel also increases the atmospheric corrosion resistance of the steel.
Chromium (Cr): Effect of Alloying elements in Steel
Chromium is a very valuable alloying element in steel making and it gives stainless steels it’s main corrosion resistance. Stainless steel are alloyed with minimum 10% chromium. Also, higher the amount of chromium in stainless steel, higher the corrosion resistance of the material. Chromium work as a ferritic stabilizer.
Chromium has little effect on steel’s strength but increases the steel’s hardening capacity. It increases the steel’s resistance to scale/oxide formation when heated to elevated temperatures, thus a primary alloying element for high temperature material such as Cr-Mo steels.
Also, it combines with carbon to form chromium carbides that are more stable than cementite, i.e., they do not break down with time at elevated temperature applications. Chromium helps to maintain the steel’s strength and reduces its flow (creep) at higher temperatures and for longer periods of time.
Molybdenum (Mo) effect on steel
Molybdenum additions minimize the damage from pitting and crevice corrosion by altering the characteristics of the surface passive film. Molybdenum is the main alloying element added to increase the resistance to chloride attack.
Nickel alloys containing in excess of 25% molybdenum have excellent resistance to hydrochloric acid (eg UNS N10675 and N10629). Molybdenum also provides appreciable high temperature solid solution strengthening and dispersion hardening through its in-fluence on the carbides present.
Higher molybdenum alloys containing chromium must also have higher nickel to minimize the possibility of sigma phase formation. Molybdenum is an important alloying element in Cr-Mo steel as it improve creep properties in steel.
Vanadium (V) effect on steel
Vanadium forms carbides & nitrides & promotes the ferrite in alloy microstructure. Vanadium is added for strength and toughness via grain refinement in as-rolled (control) as well as normalized steels.
It helps by retaining higher hardness and strength after tempering in quenched and tempered steels. Also added in some steels meant for elevated temperature applications such as Cr-Mo-V steels for reactors.
It gives rise to the hardness in martensitic steels because of its effect on carbide present in the steel. It also give rise to tempering resistance. Vanadium is only added in type of stainless steels alloys that could be hardened.
Niobium (Nb) effect on steel
Niobium also known as Columbium in U.S. is a strong carbide former & ferrite stabilizer. Similar to titanium, it support a ferritic microstructure. Niobium is used as a precipitation hardening addition, forming the coherent close packed hexagonal precipitate Ni3Nb33, sometimes referred to as y”.
It requires the presence of iron. Without iron a non-coherent phase of the same composition is formed that does not give hardening although some degree of grain refining may be obtained.
In smaller amounts niobium is used as a carbide stabilizer to help minimize or eliminate sensitization corrosion ( as it easily combine with carbon and prevent the formation of chromium carbide in stainless steel). It is also believed to increase erosion resistance. The presence of niobium in the alloy also assists in limiting carburization. It is added in amounts of 1 to 1.75% to the ‘HP’ type of alloy for this objective.
Copper (Cu) effect on steel
Copper is added to improve the corrosion resistance and steel strength. Copper promotes an austenitic microstructure. The effects of copper on toughness and hardening capacity are small. It increases the atmospheric corrosion resistance of the steel. The total amounts of copper added are small to prevent hot shortness in steel.
Boron (B) effect on steel
Boron is added to relatively low carbon steels in very small amounts to increase the hardening capacity of steels meant to be quenched and tempered. Boron is a very strong strengthening agent when used in combination with molybdenum, titanium or vanadium.
Nitrogen (N) effect on steel
Nitrogen is an alloying element commonly used with stainless steel. It is found to increase the stability of the austenite phase and make it less likely to have intermediate phase precipitates, e.g. sigma phase, increase the tensile and proof stresses without decreasing the ductility and increase the resistance to pitting and crevice corrosion.
Excess nitrogen and this will be for values in excess of less than 0.1-0.2%, depending on the alloy, will lead to porosity.
Aluminum (Al) effect on steel
Aluminum addition in sufficient quantity, improves the oxidation resistance in alloys. Due to this effect it is added in some heat-resistant materials for this objective. Aluminum addition in the precipitation hardening type steels, make intermetallic compounds to increase the aged condition alloy strength.
Titanium (Ti) effect on steel
This is added with or without aluminum to form the precipitation hardening phase Ni3(A1,Ti). It also acts as a solid solution strengthener Titanium is a carbide stabilizer and can thus be used to minimize, or eliminate, sensitization and the possibility of subsequent intergranular corrosion.
Titanium is a ferrite stabilizer and also a carbide binder in austenitic stainless steel (e.g. SS321). Ti is added to reduce the free carbon to combine with chromium so that sensitization can be avoided.
Cobalt (Co) effect on steel
Cobalt can give some improvement through the normally expected solid solution strengthening when any alloying element is in solid solution. It also minimizes, to some extent, damage through carburizing because of its higher solubility for carbon than nickel.
Cobalt assists in raising the y’ [Ni3(A1,Ti)] solution temperature and thus lowers the solubility of aluminum and titanium in the matrix and thus permits a greater proportion of precipitated y’ at higher temperatures, thus giving improved high-temperature strength. Cobalt also tends to make the distribution of this precipitate finer
Sulfur (S) effect on steel
Sulfur must always be kept low because of the possibility of forming the low melting point Ni—Ni3 S2 eutectic.
Because Sulphur will segregate to the grain boundaries, due to the low melting point phase, there is also the probability of inter-granular corrosion. Sulfur improves machinability in steel.
Tungsten (W) effect on steel
tungsten increases hardness particularly at elevated temperatures due to stable carbides, refines grain size. Tungsten is added to special grades such as Alloy 686, and Super Duplex grade 4501, which is a highly corrosion-resistant material.
Similar Posts
- Stainless Steel vs other materials-What are the advantages?
- What are Black Metals: Best for Jewelry & Artist
- Which Metals are Magnetic and not Magnetic?
- How to Identify Metals for Welding: Complete Guide
- How Does Cast Iron Compare to Other Types of Cast Iron?
- What Is the Difference Between Heat-treatable And Non-heat Treatable Alloys?