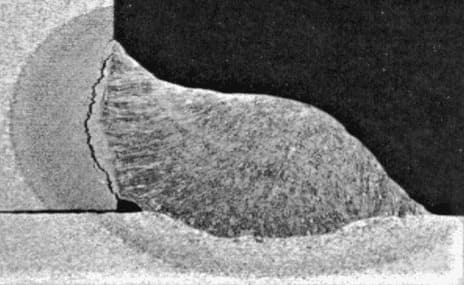
what is intergranular corrosion?
Intergranular corrosion, also known as intercrystalline corrosion, is a form of corrosion that occurs along grain boundaries in metals. It is primarily caused by the preferential dissolution of one component in a multiphase material, resulting in the localized attack along the grain boundaries.
This type of corrosion is caused by the preferential dissolution of the metal at grain boundaries resulting from the presence of impurities. It can be caused by sensitization and can lead to cracking and other serious damage in susceptible materials.
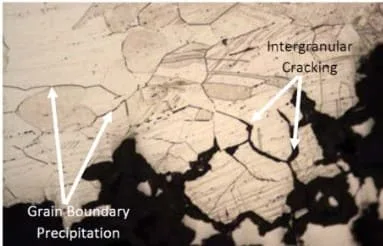
Intergranular corrosion generally results from sensitization to high temperatures during welding processes, resulting in chromium depletion at grain boundaries and other microstructural changes.
This depletion creates regions where there is an increase in susceptibility to various forms of localized attack such as pitting or crevice corrosion. The effects can range from discoloration and minor surface roughening to significant material loss and failure due to cracking or embrittlement
“Intergranular corrosion is also known as IGC or intergranular attack-IGA.“
Which metals are affected by Intergranular Corrosion?
Intergranular corrosion includes many metals such as aluminum, stainless steel, nickel alloys, etc.
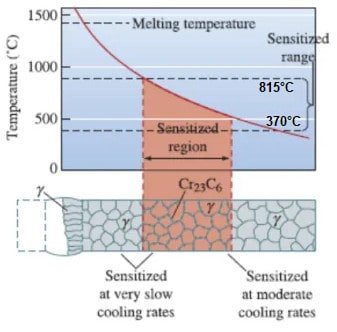
Intergranular attack in austenitic stainless steels is commonly caused by the precipitation of chromium carbides (Cr23C6) at the boundaries between grains. This leads to a localized depletion of chromium, forming a narrow region along the grain boundary. This phenomenon is known as sensitization, as depicted in Figure below.
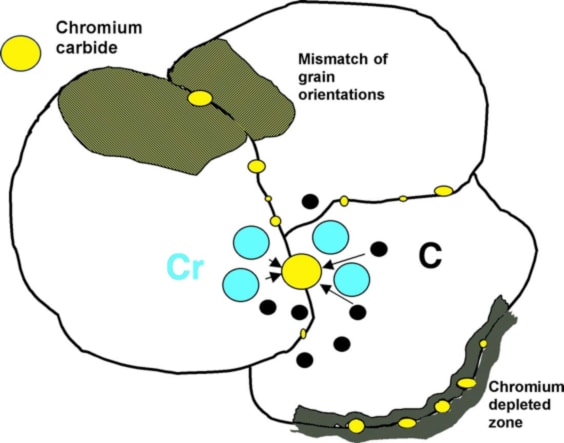
Sensitization involves the formation of chromium carbides at grain boundaries, resulting in a reduced chromium content in the immediate vicinity of the grain boundary.
intergranular corrosion causes
Intergranular corrosion is a type of corrosion that occurs along the grain boundaries of metals and alloys. This type of corrosion affects not only the surface area but can also affect the internal structure of a material and weaken its overall strength.
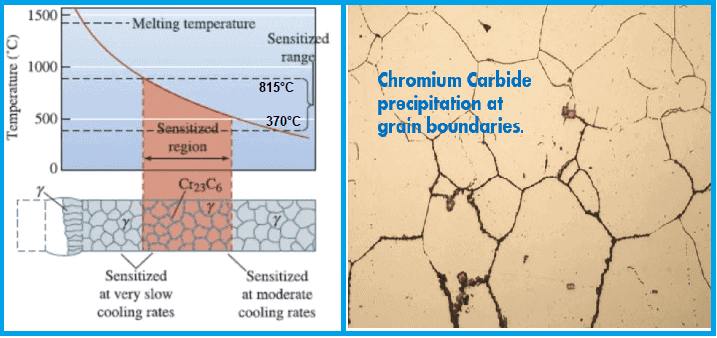
It is often caused by using improper heat treatment processes or exposing metals to certain environmental conditions like sensitization range where corrosion preventive alloying elements depleted as their carbides.
Factors increasing the risk of Intergranular corrosion are:
- Carbon content: Higher carbon content, higher risk of intergranular corrosion.
- Temperature Range: Within 650-700 °C for austenitic materials,
- Sensitization of time: the maximum degree of sensitization occurs after about 10,000 minutes,
- Grain size: Fine grains are less prone to IGC compared to coarse grains,
- Strain hardening: increases the deposition area of chromium carbides,
- Addition of alloying elements: The addition of titanium or niobium in percentage contributes to the formation of titanium or niobium carbide (since it is most closely related to carbon), which stabilizes the grain boundary and leaves intact the chromium present in the alloy.
The primary cause of intergranular corrosion is sensitization in stainless steel.
What Causes Intergranular Corrosion?
Intergranular corrosion (IGC) occurs when specific metals and alloys are exposed to temperatures ranging between 425°C and 870°C (887°F to 1598°F). These temperature ranges are commonly encountered during processes such as welding, heat treatment, or operation in high-temperature environments.
How does intergranular corrosion occur?
Intergranular corrosion is a form of corrosion that affects metal alloys, particularly stainless steel and aluminum alloys. It occurs when the grain boundaries are corroded away and can be caused by many different factors. Understanding how intergranular corrosion manifests is essential for designing structures with greater durability and longevity.
Intergranular corrosion can be caused by a variety of factors, including improper heat treatment, incorrect alloy composition, or contamination with certain chemicals. This form of corrosion takes place when the grains in a material become exposed to an environment where they are more prone to oxidation.
This happens due to differences in composition among the grains, causing them to corrode at different rates.
Poor production methods like welding or casting may also create areas where IGC is more likely to occur. Additionally, environmental factors such as temperature variations can cause one grain boundary to oxidize faster than another resulting in IGC.
ASTM intergranular corrosion test
The ASTM Intergranular corrosion test, ASTM A262, is a widely used standard for testing the susceptibility of stainless steel to intergranular corrosion.
The ASTM A262 test consists of five different steps: sample preparation, solution preparation, testing procedure, evaluation criteria and reporting requirements. During sample preparation, samples must be cut from the material and machined into the appropriate shape for testing.
The acid solution is then prepared using either nitric acid or sulfuric acid mixed with acetic anhydride at various temperatures depending on the grade of stainless steel being tested.
How to detect intergranular corrosion?
Intergranular corrosion is a type of corrosion that occurs on the grain boundaries of metal alloys, usually stainless steel. It can lead to serious damage if left unchecked. Fortunately, there are a few methods that can be used to detect intergranular corrosion in metals before it causes irreversible harm.
First, visual inspection is one of the most straightforward methods for identifying intergranular corrosion. It involves using a microscope or magnifying glass to examine surfaces for signs of pitting or etching along grain boundaries.
Additionally, mechanical testing such as hardness testing and tensile strength testing can be used to measure changes in material properties that may indicate intergranular corrosion.
Another way to detect intergranular corrosion is through chemical analysis such as electrochemical tests or X-ray diffraction analysis.
How can intergranular corrosion be prevented?
Intergranular corrosion can be prevented using:
- Using stabilizing grades alloyed with Niobium (Nb) such as AISI 347 or with Titanium (Ti) such as AISI 321.
- Using steel with low carbon content.
- Apply solution annealing after welding.
- Welding using low heat input and controlling interpass temperature.
- Avoiding service temperature in sensitization range.
- Additional increase in chromium concentration.
- The introduction of molybdenum, which slows down the process of release of chromium carbides.
- Using low carbon welding filler wire rod (e.g., ER308L instead of ER308).
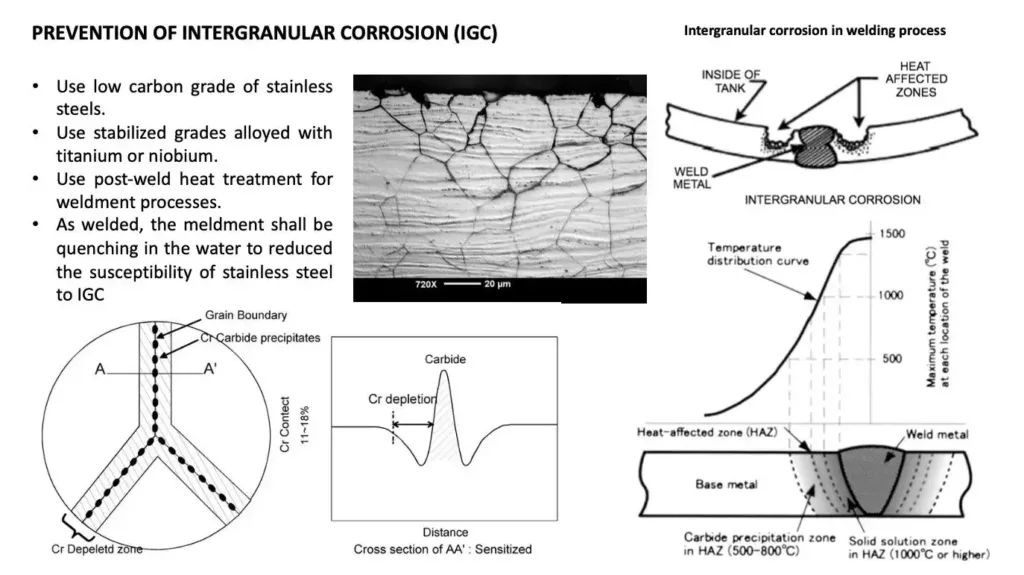
Additionally, proper annealing procedures should be followed for stainless steel materials in order to avoid this form of corrosion. The annealing process helps to dissolve chromium carbides at grain boundaries which makes them less vulnerable to intergranular attack.
The inclusion of stabilizing elements such as Ti, Nb (Cb), and Ta can enhance the resistance to sensitization, particularly during prolonged exposures within the critical temperature range during service. These elements have a tendency to form carbides that are more thermally stable compared to chromium carbide within the temperature range of 2250 to 1450°F.
As the alloy cools down from high temperatures, the carbon combines with the stabilizing elements, making it unavailable for the precipitation of chromium carbide in the lower temperature range of 950 to 1450°F, which is prone to sensitization. Common examples of stabilized austenitic grades that utilize these elements include Type 321, 347, 20-Cb3, and 316Ti.
Table below shows the carbide precipitation temperature range for different types of carbide in stainless steel.
| Temperature Range | Precipitation Reactions |
|---|---|
| Melting point – 2250 °F | Niobium (Columbium) carbide dissolves Chromium carbides dissolves |
| 2250 to 1450 °F | Columbium carbide precipitates Chromium carbides dissolves |
| 1450 to 950 °F | Chromium carbides precipitates |
| 950 to 70 °F | No reaction |
How to reverse the effect of Intergranular corrosion?
Fortunately, employing heat treatments can often effectively address the problem and restore the metal’s structure to a state that closely resembles its original condition.
In certain cases, the application of solution-annealing (also known as quench-annealing or solution-quenching) proves to be a reliable approach for reversing intergranular corrosion damage specifically in austenitic stainless steels.
This process involves subjecting the metal to elevated temperatures, typically ranging between 1,060°C and 1,120°C (1940°F and 2048°F).
Once the metal reaches the desired temperature, it undergoes rapid cooling through water quenching, facilitating the solidification of the grain structure.
Unfortunately, this method presents challenges when dealing with large assemblies and does not offer long-term protection to pipes or other components against potential damage during subsequent welding or repair operations.
What is intergranular corrosion of austenitic stainless steel?
Intergranular corrosion (IGC) is corrosion of austenitic stainless such as AISI 304, 310 and 316 spreading along the grain boundaries due to the formation of chromium carbides along the grain boundaries and depletion of adjacent volumes with chromium.
The most common carbide Cr23C6, which greatly reduces the ductility and toughness of the metal. Intergranular corrosion of the welded joint most often occurs in the base metal near the weld (in HAZ), as well as at the place of fusion of the base metal with the welded and in the welded metal.
Intergranular Corrosion runs along the grain boundaries, which leads to the formation of deep cracks. This can result in structure failure.
Can you see intergranular corrosion?
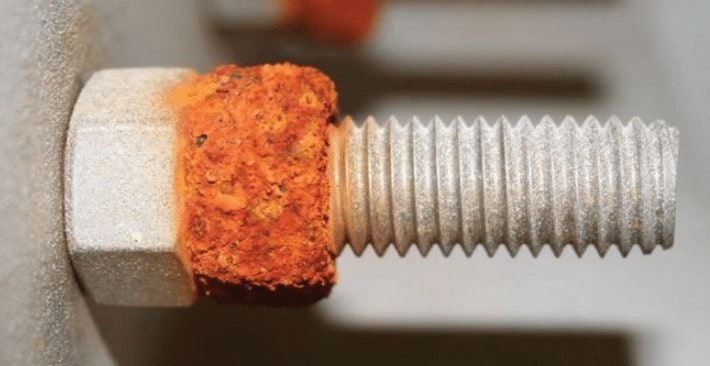
External intergranular corrosion (IGC) is visible as black spots. For internal IGC, it needs to be checked via metallography.
Correct. Intergranular corrosion (IGC) primarily affects the structure of the metal at a microscopic level and may not manifest visible signs of attack on the surface. Unlike other types of corrosion that often result in visible changes such as rust, pitting, or surface irregularities, IGC occurs along the grain boundaries of the material, making it difficult to detect with the naked eye.
Specialized testing methods, such as metallographic examination or chemical testing, are typically required to identify and assess the extent of intergranular corrosion.
Intergranular vs Transgranular corrosion
ntergranular corrosion (IGC) and transgranular corrosion (TGC) are two types of corrosion caused by exposure to aggressive environments. IGC is a form of localized corrosion that occurs along grain boundaries, while TGC is a more generalized form of attack on the entire grain or crystal structure.
The main difference between IGC and TGC lies in the way each type of corrosion attacks the material’s surface. IGC occurs at the interface between grains, leading to cracks and crevices that reduce mechanical strength; whereas with TGC, no distinct boundaries exist as the entire grain structure is attacked simultaneously. This often results in pits that extend deep into the material’s surface over time.
Intergranular Corrosion of Ferritic Stainless Steels
While intergranular attack in ferritic stainless steels shares similarities with that in austenitic stainless steels, there are noteworthy differences. In ferritic grades, sensitization is caused by the precipitation of both chromium carbides (Cr23C6) and chromium nitrides (Cr2N) due to the low solubility of nitrogen in the ferritic crystal structure.
Sensitization in ferritic grades occurs during the cooling process from high temperatures (>1700 °F). At these elevated temperatures, carbides and nitrides dissolve into the steel’s structure, and upon cooling, they precipitate at grain boundaries, resulting in chromium depletion.
The rapid diffusion rates in the ferrite structure make it challenging to cool the steel quickly enough to prevent the precipitation of carbides and nitrides at grain boundaries. To mitigate this, commercial ferritic grades limit the levels of carbon and nitrogen while incorporating stabilizing elements such as titanium (Ti), tantalum (Ta), or niobium (Nb).
In the event that sensitization has occurred in a ferritic stainless steel, the condition can be “healed” by diffusing chromium back into the depleted areas. This healing process involves holding the material at temperatures between 1100 and 1200 °F for several hours. ASTM A763 provides test methods specifically designed to detect susceptibility to intergranular attack in ferritic stainless steels.
Intergranular corrosion of Aluminum
Intergranular corrosion of aluminum alloys also occurs when they are heated during operation.
In most cases, intergranular corrosion of aluminum products is noted in areas where there are microscopic pores and small cracks. Less commonly, damage begins in pitting’s. They are formed between the boundaries of grains.