Stainless steel possesses a unique property called passivity, which grants it superior corrosion resistance. This characteristic is attributed to an extremely thin transparent film that forms on the surface of stainless steels.
In this blog post, we will explore the process of passivation, its significance in improving corrosion resistance, and the methods employed to achieve effective passivation of stainless steel. Additionally, we will discuss related topics, such as the importance of surface cleanliness and the role of passivation in maintaining surface quality.
What is Passivation of stainless steel?
Passivation of stainless steel is a chemical treatment process that involves the use of a specialized acid formulation.
Its primary purpose is twofold:
- To eliminate free iron and other surface contaminants from the stainless steel and
- To facilitate the formation of a passive chromium/nickel oxide layer.
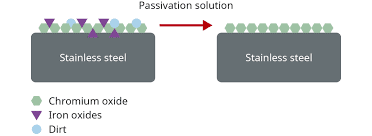
This oxide layer serves as a protective barrier, effectively preventing or reducing the occurrence of future corrosion. By removing impurities and promoting the formation of a passive film, passivation enhances the corrosion resistance of stainless steel surfaces.
Principle of Stainless Steel Passivation
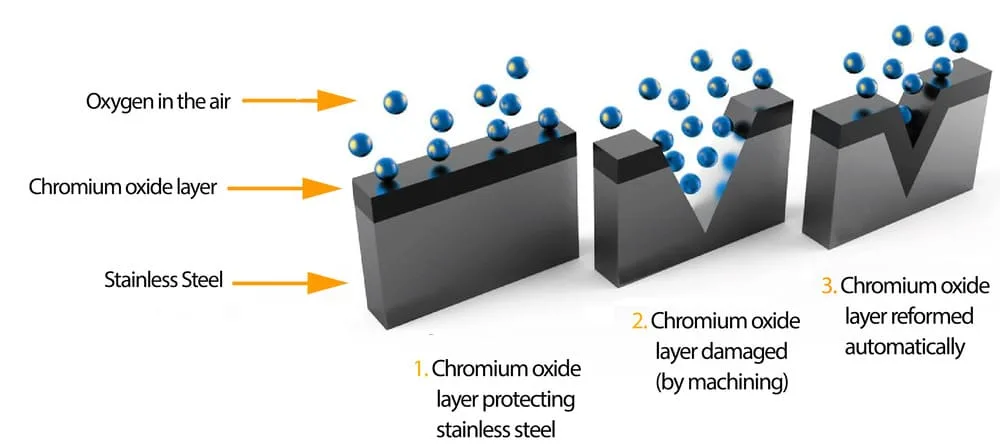
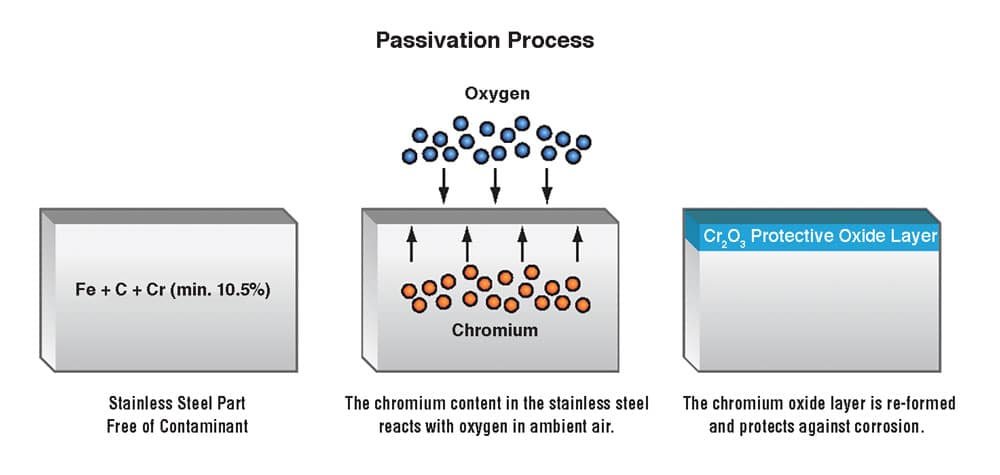
The process of stainless steel passivation involves the gradual formation of a protective layer on the steel surface through the reaction of chromium with oxygen in the air.
—This reaction produces chromium oxide, which renders the steel unreactive or “passive.” —
This passive layer is extremely beneficial for equipment used in various industries such as food, beverage, dairy, pharmaceutical, cosmetics, and cannabis processing.
The chemical equation representing the reaction is:
- 4Cr + 3O2 → 2Cr2O3
Although this protective layer is invisible and only a few molecules thick, it acts as a barrier that prevents oxygen and moisture from reaching the underlying iron.
Without this barrier, the iron would oxidize and rust, resulting in an undesirable yellow, orange, or red color. Rust weakens the steel, making it prone to flaking, which poses contamination risks and safety concerns.
Why Passivation of Stainless Steel is done?
The passive film present on stainless steel surfaces plays a critical role in imparting corrosion resistance. This film, though thin, is tenacious, uniform, and stable. It resembles the passivity typically associated with noble or inert metals. Stainless steels owe their exceptional resistance to corrosion to the presence of this passive film.
During the manufacturing process of stainless steel products, the surface of the material can become contaminated with free iron. This iron contamination originates from the steel cutting, stamping, and forming tools employed in production.
Additionally, polishing or blasting operations that involve the shared use of media between mild steel and corrosion-resistant steel grades can introduce free iron onto the stainless steel surface. The presence of free iron leads to oxidation, resulting in the visible formation of rust on the product’s surface.
How passivation layers form on Stainless Steel?
The passive film on stainless steel surfaces forms spontaneously in the presence of oxygen, whether in the air or when immersed in solutions. It has the ability to repair itself if damaged, provided sufficient oxygen or oxidizing elements are available.
This inherent property makes stainless steel an ideal material for various applications where corrosion resistance is paramount.
So how does chromium prevent corrosion?
Chromium prevents corrosion in stainless steel by forming a thin, invisible layer of chromium oxide on the surface. This layer acts as a protective barrier, preventing the metal from reacting with the environment and corrosive substances.
It is chemically stable, self-healing when damaged, and acts as a physical barrier against water, oxygen, and chemicals, reducing the likelihood of corrosion.
Passivation Process
The primary method for passivating stainless steel involves exposing a clean surface to air. However, practical evidence suggests that passivity and corrosion resistance can be further enhanced by forming the passive film through the action of oxidizing acid solutions.
Among these solutions, nitric acid is commonly employed for passivation treatments. Notably, nitric acid does not corrode stainless steel, alter critical dimensions, or remove heat tint or embedded iron.
What is Chemical Passivation?
Chemical passivation is a surface treatment process used to enhance the corrosion resistance of metal, particularly stainless steel. It involves the use of chemicals to create a protective oxide layer on the surface of the metal. The passivation process typically involves the immersion or application of a passivating solution, which is often based on nitric acid or citric acid.
During chemical passivation, the passivating solution reacts with the metal surface, removing any impurities, contaminants, or free iron that may be present. This helps to create a clean and passive surface. The passivating solution also facilitates the formation of a thin, transparent oxide layer, primarily composed of chromium oxide, on the metal surface. This oxide layer acts as a barrier, protecting the underlying metal from corrosion by preventing direct contact with corrosive agents, such as oxygen and moisture.
Chemical passivation is commonly used in various industries, including aerospace, automotive, medical, and food processing, where corrosion resistance is crucial. It is an effective method to improve the longevity and durability of metal components by reducing the risk of corrosion and maintaining the desired surface properties.
Nitric Acid Passivation Procedure
The most commonly utilized chemical method for passivating a stainless steel surface involves the use of nitric acid. Nitric acid is a strong mineral acid that swiftly dissolves iron compounds and other trace metals present on the surface. Additionally, it acts as a potent oxidizer, activating the chromium oxide layer at the same time.
“The standard nitric acid passivating solution consists of 10 to 15 percent nitric acid (HNO3) in water. “
For optimal results, the solution is typically used at temperatures of 150°F (65°C) for austenitic stainless steels (300 series) and 120°F (50°C) for ferritic and martensitic stainless steels (400 series). The immersion time is approximately 30 minutes, followed by thorough water washing.
The recommended conditions for applying nitric acid passivation are as follows:
Time: 3 – 4 hours
Temperature: Up to 80°C/175°F
Concentration: 20% to 50% nitric acid by volume
Citric Acid Passivation Procedure
An alternative method for passivating stainless steel involves the use of citric acid. Citric acid is capable of effectively removing iron and its compounds from surfaces. Compared to nitric acid, it offers several advantages such as being safer to use, biodegradable, and producing fewer concerns regarding effluent. Additionally, citric acid is commonly used as a food ingredient.
However, it is important to note that citric acid is not an oxidizer and does not achieve the second step of the traditional passivation process. Instead, it relies on natural air oxidation to form the protective oxide layer.
The recommended application conditions for citric acid passivation are as follows:
Time: 5 hours
Temperature: Ambient to moderate heating
Concentration: 12% by weight
Alternative Passivation Methods
In situations where full immersion in a hot passivating solution is not feasible, swabbing with a cold acid solution is often employed. Lower concentrations and temperatures of acid make the handling and application easier. However, longer contact times are usually required to achieve the desired passivation effect.

Passivation for Different Stainless Steel Grades
Passivation requirements may vary depending on the stainless steel grade. The following guidelines are typically recommended:
- For austenitic stainless steels (300 series): 15% nitric acid at 65/80°F (20/25°C) for 30 to 90 minutes.
- For ferritic stainless steels (400 series): 12% nitric acid at 65/80°F (20/25°C) for 30 to 45 minutes.
Application Techniques
Swabbing the acid solution onto the stainless steel surface with sponges, soft paintbrushes, or fine nylon pads is a common application method.
Continuous swabbing ensures adequate contact over the required time period. In cases where localized treatment is needed, proprietary passivating pastes are available and can be used as per the supplier’s recommendations.
How to Verify Passivation Solution Strengths?
To verify the strength of a passivation solution, the acid concentration is typically measured using a laboratory technique called titration. This process involves adding a known concentration of a solution (titrant) to a set volume of the passivation solution until a visible color change indicates the reaction has reached equilibrium.
The quantity of titrant required for neutralization is then used to determine the strength of the passivation solution, ensuring it meets the specified acid concentration range according to industry standards.
ASTM Standards for Stainless Steel Passivation
- A380/A380M-17 – Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems
- Subcommittee: A01.14 on Methods of Corrosion Testing
- Description: This standard provides recommendations and precautions for the cleaning, descaling, and passivation of new stainless steel parts, assemblies, equipment, and installed systems. It covers various aspects such as design considerations, pre-cleaning and de-scaling procedures, treatment of weld areas, inspection and cleanliness standards, and precautions to avoid specific issues.
- ASTM A967 / A967M-17 – Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts
- Description: This standard specifies the requirements for chemical passivation treatments of stainless steel parts. It covers passivation by immersion treatment using nitric acid, citric acid solutions, and electropolishing. The standard outlines the proper process steps, defines the desired appearance of the passivated surface, and includes tests that should be conducted to demonstrate the successful passivation of the stainless steel parts.
- AMS 2700 is an aerospace industry standard for quality and consistency in passivation processes. It specifies requirements for surface preparation, passivation, and testing of stainless steel parts used in aerospace applications.
These standards provide guidance and specifications for ensuring effective passivation of stainless steel parts and systems, promoting corrosion resistance and maintaining the desired surface characteristics.
Standards for Passivation of Stainless Steel
Passivation of stainless steel involves employing specific acid formulations to remove free iron and surface contaminants, while promoting the formation of a protective oxide layer. Here, we explore the passivation techniques specified by ASTM A967, AMS 2700, and QQ-P-35.
ASTM A967:
Five nitric acid methods are outlined:
- Nitric 1: 20-25 v% Nitric Acid, 2.5 w% Sodium Dichromate, 120-130F, 20 Mins minimum
- Nitric 2: 20-45 v% Nitric Acid, 70-90F, 30 Mins minimum
- Nitric 3: 20-25 v% Nitric Acid, 120-140F, 20 Mins minimum
- Nitric 4: 45-55 v% Nitric Acid, 120-130F, 30 Mins minimum
- Nitric 5: Customizable combinations of temperature, time, and acid, with or without accelerants, inhibitors, or proprietary solutions to meet the specified test requirements.
For citric acid passivation, four methods are provided:
- Citric 1: 4-10 w% Citric Acid, 140-160F, 4 Mins minimum
- Citric 2: 4-10 w% Citric Acid, 120-140F, 10 Mins minimum
- Citric 3: 4-10 w% Citric Acid, 70-120F, 20 Mins minimum
- Citric 4: Customizable combinations of temperature, time, and citric acid concentration, with or without additional chemicals to enhance cleaning, accelerants, or inhibitors, meeting the specified test requirements. Immersion bath pH should be controlled at 1.8-2.2.
Five testing methods are provided for passivation validation:
- Practice A: Water Immersion Test
- Practice B: High Humidity Test
- Practice C: Salt Spray Test
- Practice D: Copper Sulfate Test
- Practice E: Potassium Ferricyanide-Nitric Acid Test
AMS 2700:
Both nitric and citric acid methods are covered in AMS 2700.
- Method 1: Passivation in Nitric Acid, with various types specifying different conditions and concentrations of nitric acid and sodium dichromate.
- Method 2: Passivation in Citric Acid, with recommended citric acid concentrations and temperature/time ratios. Additional wetting agents and inhibitors can be used.
Various tests are defined within AMS 2700 to assess corrosion resistance. However, certain alloys, like high carbon martensitic steels, may be exempt from specific testing requirements due to potential false positives.
QQ-P-35:
QQ-P-35 covers only nitric acid passivation services and provides four active types:
- Type II: 20-25 v% Nitric Acid, 2-2.5 w% Sodium Dichromate, 120-130F, 20 Mins minimum
- Type VI: 25-45 v% Nitric Acid, 70-90F, 30 Mins minimum
- Type VII: 20-25 v% Nitric Acid, 120-150F, 20 Mins minimum
- Type VIII: 45-55 v% Nitric Acid, 120-130F, 30 Mins minimum
Four testing methods are specified for passivation validation in QQ-P-35:
- 4.4.1.1: Water Immersion Test
- 4.4.1.2: High Humidity Test
- 4.4.2.1: Salt Spray Test
- 4.4.2.2: Copper Sulfate Test
Passivation Steps for Stainless Steel
Passivation Steps for Stainless Steel:
- Thorough Cleaning:
- Remove grease, coolant, and other debris from the surface using a degreaser or cleanser.
- Carefully wipe off machining chips or shop dirt from the part.
- Preparation for Passivation:
- Ensure the part is free from any residual cutting fluid or grease to prevent excessive oxidation and surface carburization.
- If necessary, clean the part with a degreaser or cleanser to remove any traces of cutting fluid.
- Selection of Passivating Bath:
- Choose the appropriate passivating bath based on the stainless steel grade and acceptance criteria.
- Options include nitric acid passivation, nitric acid with sodium dichromate passivation, or citric acid passivation.
- Passivation Process:
- After thorough cleaning, immerse the stainless steel part in the selected passivating bath.
- Follow the recommended parameters for temperature, concentration, and immersion time according to the stainless steel grade.
- Nitric Acid Passivation:
- For chromium-nickel grades (300 Series), immerse the part in a 20% by vol. nitric acid bath at 120/140°F (49/60°C) for 30 minutes.
- For straight chromium grades (12-14% Chromium) and high carbon-high chromium grades (440 Series), use a 20% by vol. nitric acid bath with 3 oz. per gallon (22 g/liter) of sodium dichromate at 120/140°F (49/60°C) for 30 minutes.
- Alternatively, for these grades, a 50% by vol. nitric acid bath at 120/140°F (49/60°C) for 30 minutes can be used.
- Passivation for Free-Machining Stainless Steels:
- Degrease the parts and soak them for 30 minutes in a 5% solution of sodium hydroxide at 160/180°F (71/82°C).
- Rinse the parts thoroughly with water.
- Immerse the parts for 30 minutes in a 20% by vol. nitric acid solution with 3 ounces per gallon (22 g/liter) of sodium dichromate at 120/140°F (49/60°C).
- Remove the parts from the bath, flush them with water, and immerse them in the sodium hydroxide solution for another 30 minutes.
- Rinse the parts again with water and dry them, completing the A-A-A method of passivation.
- Citric Acid Passivation:
- Citric acid passivation is an alternative to mineral acid passivation and sodium dichromate-containing solutions.
- Follow the recommended procedure for citric acid passivation, ensuring the right concentration, temperature, and immersion time to avoid flash attack.
- Safety and Environmental Considerations:
- Citric acid passivation is considered environmentally friendly and safe for handling.
- Fabricators using mineral acid passivation without safety issues may continue with their current process if it yields satisfactory results.
Stainless Steel Passivation vs Pickling
| Criteria | Stainless Passivation | Pickling |
|---|---|---|
| Purpose | Create a new protective layer | Remove a damaged layer and restore corrosion resistance |
| Objective | Enhance corrosion resistance | Eliminate heat tint and restore original form |
| Method | Chemical treatment (nitric acid or citric acid) | Chemical treatment (nitric acid or hydrofluoric acid), electropolishing, or mechanical removal |
| Chemical Aggressiveness | Milder acids (nitric acid or citric acid) | More aggressive acids (nitric acid or hydrofluoric acid) |
| Layer Formation | Creates a new chromium oxide layer | Removes damaged layer to expose original corrosion-resistant alloy |
| Focus | Enhancing existing protective properties | Removing damaged heat tint layer |
| Result | Reinforces corrosion resistance | Restores original corrosion-resistant form |