What is Galvanic Corrosion?
Galvanic corrosion refers to the phenomenon that occurs when two different metals or alloys are exposed to a corrosive environment or are regularly connected through moisture. In such conditions, each metal develops a corrosion potential.
The metal with higher nobility becomes the cathode, while the metal with higher reactivity becomes the anode.
Consequently, a measurable current can flow between the anode and the cathode. This results in an accelerated corrosion rate for the anode, known as galvanic corrosion, while the corrosion rate of the cathode decreases.
What is required for galvanic corrosion?
Similar to a Fire triangle that relates to fire to occur, A Galvanic corrosion requires the presence of three essential elements:
- Metals with different corrosion potentials: Two distinct metals or alloys must be involved, each with its own inherent corrosion potential.
- Direct electrical contact between metals: There should be a direct, uninterrupted contact between the two metals, allowing for the flow of electrical current.
- Conductive electrolyte solution: A conductive electrolyte, such as water or moisture from sources like immersion, condensation, rain, or fog, must be present to regularly connect and dampen the two metals, establishing a conductive path.
If any of these elements are missing, galvanic corrosion cannot occur. For instance, if the direct contact between the metals is prevented by using a plastic washer or a layer of paint, or if there is an interruption in the conductive path, galvanic corrosion will not take place, and each metal will corrode at its usual rate in the given service environment.
Figure 1 provides examples of situations that do not fulfill all the requirements for galvanic corrosion.
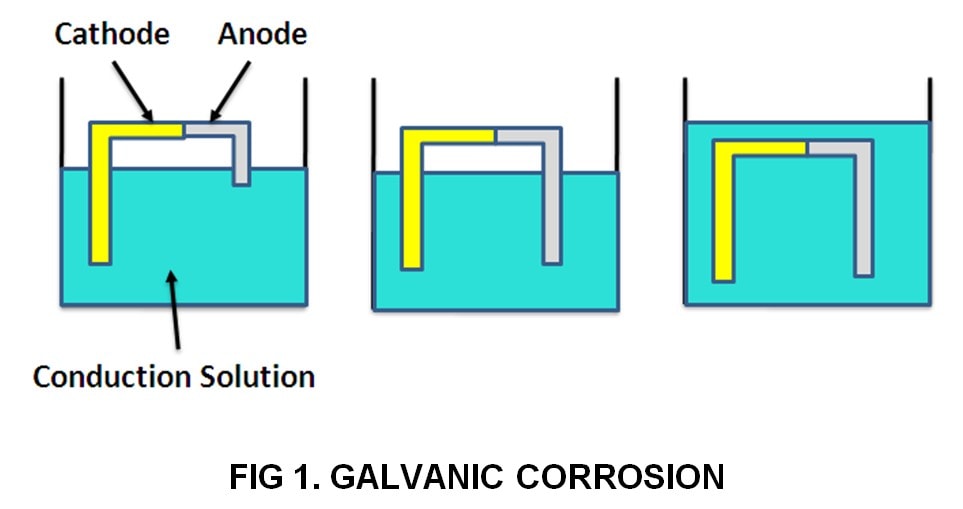
1. Metals with different corrosion potentials
Galvanic corrosion can occur when two different types of metals come into contact. The metal with a more electronegative potential, or in the case where both metals are electronegative, the more electronegative metal, becomes the anode in the galvanic couple.
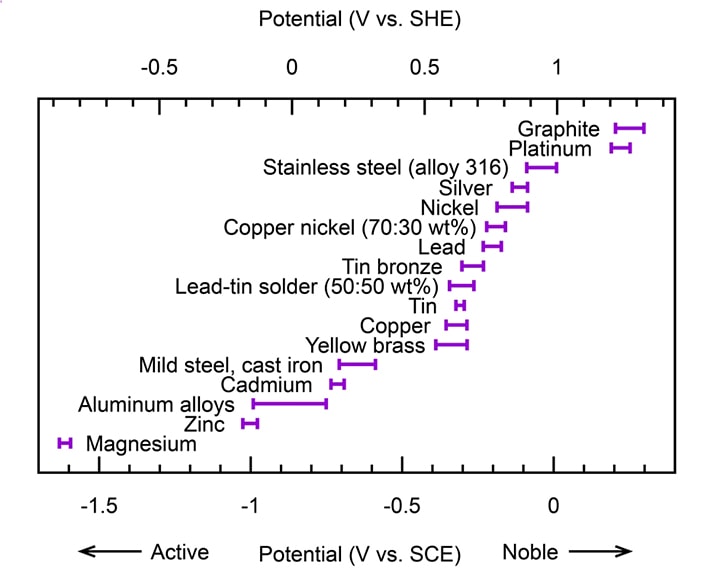
To determine which metal will experience galvanic corrosion, it is necessary to compare the dissolution potentials of the most commonly used metals and alloys. It has been observed that galvanic corrosion typically occurs when there is a potential difference of at least 100 mV between the two metals. The intensity of galvanic corrosion is not directly related to the difference in potential between the metals.
For example, based on the potential ranking of aluminum and its alloys, in most situations where aluminum is in contact with other common metals, aluminum will act as the anode in the resulting galvanic cell. Therefore, if the conditions are favorable, aluminum is more likely to experience galvanic corrosion.
2. Direct electrical contact between metals
Electrical continuity between the metals can be established through either direct contact between the two metals or by using a connection method such as bolts. In both cases, the goal is to ensure a solid and uninterrupted electrical path between the metals.
This electrical continuity is essential for the flow of current in a galvanic couple, enabling the occurrence of galvanic corrosion if other necessary conditions are also met.
Whether through direct metal-to-metal contact or the use of connecting elements like bolts, establishing electrical continuity is crucial for the initiation and progression of galvanic corrosion.
3. Conductive electrolyte solution
For galvanic corrosion to occur, the contact area between the metals must be wetted by an aqueous liquid to facilitate ionic conduction. Without this presence of an aqueous solution, galvanic corrosion cannot take place.
In some specific cases, such as the tank of an electrical transformer, where aluminum conductors and copper conductors are immersed in an oil bath, there is no possibility of galvanic corrosion of aluminum. This is because the oil bath does not provide the necessary aqueous medium for galvanic corrosion to occur.
It is worth noting that galvanic corrosion between metals can also happen in other ionic media beyond aqueous solutions. For example, it can occur in liquid fluorine, liquid ammonia, and concentrated nitric acid, as mentioned in reference. In these cases, the presence of the respective ionic media enables the occurrence of galvanic corrosion between metals.
Science behind galvanic corrosion
The corrosion potential and the galvanic series are related to the electrochemical reactions that occur when a metal corrodes in an electrolyte. When a metal corrodes, it undergoes an anodic reaction where metal atoms separate into ions and electrons. For example, in the case of iron (Fe), the anodic reaction is:
- Fe → Fe2+ + 2e−
This reaction results in the formation of Fe2+ ions. On the other hand, there are cathodic reactions that remove electrons from the system. For instance, oxygen (O2) dissolved in the electrolyte can react to form hydroxide ions (OH−) through the following cathodic reaction:
- O2 + 2H2O + 4e− → 4OH−
In these reactions, electrons are deposited in the metal during the anodic reaction and removed from it during the cathodic reaction. A balance is achieved when the rate of electron deposition matches the rate of electron removal.
As a result of the electric charges carried by the ions and electrons, the corroding metal develops an electric potential. This potential, which arises from the simultaneous occurrence of multiple reactions, is referred to as a mixed potential or corrosion potential.
How Galvanic Corrosion takes place?
Galvanic corrosion occurs through a series of electrochemical reactions between two dissimilar metals or alloys in the presence of an electrolyte.
Here’s a step-by-step explanation of how galvanic corrosion takes place:
- Formation of an electrolyte: An electrolyte, typically a liquid such as water or moisture from the environment, is present and provides a conductive medium for ion transport.
- Contact between dissimilar metals: Two different metals or alloys come into direct contact with each other, forming a galvanic couple. One metal acts as the anode, and the other metal acts as the cathode.
- Difference in corrosion potentials: The two metals have different corrosion potentials, meaning they have different tendencies to undergo oxidation (corrosion) reactions. The metal with a higher corrosion potential becomes the anode, while the metal with a lower corrosion potential becomes the cathode.
- Anodic reaction (oxidation): At the anode, the metal undergoes oxidation, releasing metal ions into the electrolyte. This process is often referred to as the anodic reaction or anodic dissolution.
- Cathodic reaction (reduction): At the cathode, the metal acts as a site for reduction reactions. It attracts electrons from the electrolyte, where reduction reactions take place, often involving oxygen or hydrogen ions present in the electrolyte.
- Electron flow (current): Electrons flow from the anode to the cathode through a conductive path, such as a metal-to-metal connection or an external circuit. This electron flow creates an electrical current.
- Ionic conduction: The metal ions released at the anode migrate through the electrolyte toward the cathode, completing the ionic conduction process.
- Corrosion products and damage: As the anode metal corrodes, it forms corrosion products that can include oxides, hydroxides, or salts. These corrosion products may accumulate, leading to visible damage, such as pitting or erosion, on the anode metal surface.
Overall, galvanic corrosion occurs due to the electrochemical interaction between dissimilar metals, facilitated by the presence of an electrolyte and the flow of electrons and ions. The accelerated corrosion of the anode metal and the protection of the cathode metal result in the degradation of the galvanic couple.
How to prevent Galvanic Corrosion?
Galvanic corrosion can be prevented through various measures, including:
- Choosing materials with similar corrosion potentials: Opting for metals or alloys that have similar electrochemical properties reduces the likelihood of galvanic corrosion.
- Insulating the metals: Breaking the electrical connection between the metals by using insulating materials, such as gaskets, plastic washers, or coatings, helps prevent galvanic corrosion.
- Applying protective coatings: Coating both metals with protective layers helps inhibit the galvanic reaction. Maintaining a well-maintained coating on the cathode is particularly important to prevent the worsening of galvanic corrosion.
- Using spacers: Introducing non-conductive spacers of appropriate sizes between the metals physically separates them, preventing electrical contact and subsequent galvanic corrosion.
- Employing sacrificial anodes: Installing sacrificial anodes that are more anodic than the metals involved directs the corrosion towards the sacrificial anode, protecting the primary metals from galvanic corrosion.
- Utilizing corrosion inhibitors: Incorporating corrosion inhibitors into the environment helps mitigate galvanic corrosion by reducing the electrochemical reactions between the metals and the electrolyte.
If implementing the above measures is not feasible, minimizing the rate of galvanic corrosion can be achieved by ensuring a large anode-to-cathode area ratio, preferably greater than 10. Alternatively, designing the anode with an appropriate corrosion allowance can provide additional protection.
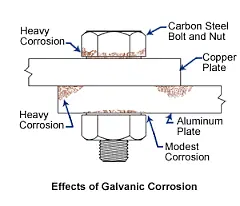
It is important to note that galvanic action can result in selective corrosion of welds in specific environments. For example, in seawater, carbon steel weld metal may be more susceptible to severe corrosion compared to the adjacent parent material, which remains unaffected. It is crucial to take appropriate preventive measures to address this vulnerability of welds to galvanic corrosion.
Galvanic corrosion table of metals in seawater
The table presents the metals and alloys listed in order from more noble (less reactive) at the top to more active (more reactive) at the bottom:
| Metal/Alloy | Symbol | Density (g/cm³) | Series |
|---|---|---|---|
| Graphite (carbon) | C | 2.26 | N/A |
| Platinum | Pt | 21.45 | Noble Metals |
| Gold | Au | 19.32 | Noble Metals |
| Stainless steel (alloy 316) | SS316 | 8.00 | Stainless Steel |
| Silver | Ag | 10.49 | Noble Metals |
| Nickel | Ni | 8.91 | Nickel |
| Copper nickel (70:30 wt%) | Cu-Ni | 8.95 | Copper-Nickel |
| Lead | Pb | 11.34 | Lead |
| Tin bronze | Cu-Sn | Varies | Bronze |
| Lead-tin solder (50:50 wt%) | Pb-Sn | Varies | Solder |
| Tin | Sn | 7.29 | Tin |
| Copper | Cu | 8.96 | Copper |
| Yellow brass | Cu-Zn | Varies | Brass |
| Chromium (plated on steel) | Cr | 7.14 | Chromium |
| Mild steel, cast iron | Fe | Varies | Steel |
| Cadmium | Cd | 8.65 | Cadmium |
| Aluminum alloys | Al | Varies | Aluminum |
| Zinc | Zn | 7.14 | Zinc |
| Magnesium | Mg | 1.74 | Magnesium |
Anodic Index of metals
| Metallurgical Category | Anodic Index (V) |
|---|---|
| Gold (Au), solid and plated, Gold-platinum alloy | 0.00 |
| Rhodium (Rh) plated on silver-plated copper | 0.05 |
| Silver (Ag), solid or plated; Monel metal; High nickel-copper alloys | 0.15 |
| Nickel (Ni), solid or plated, titanium and its alloys, Monel | 0.30 |
| Copper (Cu), solid or plated; low brasses or bronzes; silver soldier; German silvery high copper-nickel alloys; nickel-chromium alloys | 0.35 |
| Brasses and Bronzes | 0.40 |
| High brasses and bronzes | 0.45 |
| 18% chromium type corrosion-resistant steels | 0.50 |
| Chromium plated; tin plated; 12% chromium type corrosion-resistant steels | 0.60 |
| Tin-plate; tin-lead solder | 0.65 |
| Lead (Pb), solid or plated; High lead alloys | 0.70 |
| Aluminum (Al) wrought alloys of the 2xxx Series | 0.75 |
| Iron (Fe), wrought, gray or malleable, plain carbon and low alloy steels | 0.85 |
| Aluminum (Al), wrought alloys other than the 2xxx Series, cast alloys of the silicon type | 0.90 |
| Aluminum (Al), cast alloys other than silicon type; Cadmium, plated and chromate | 0.95 |
| Hot-dip-zinc; galvanized steel | 1.20 |
| Zinc (Zn), wrought; zinc-base die-casting alloys; zinc plated | 1.25 |
| Magnesium (Mg) and magnesium-based alloys, cast or wrought | 1.75 |
| Beryllium (Be) | 1.85 |
Galvanic Corrosion of metals in contact
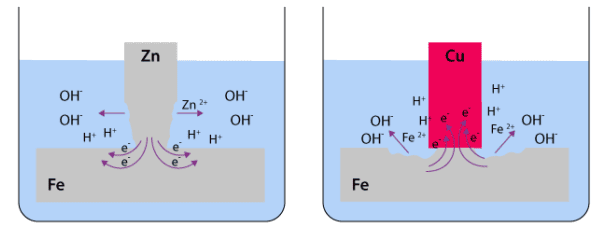
When two metals, such as silver and iron, are connected in an electrolyte, their potentials become equalized and settle at a new value between their respective corrosion potentials. Consequently, the corrosion rates of the metals are affected.
The more active metal, in this case, iron, corrodes at a faster rate compared to when it is alone, while the more noble metal, silver, experiences slower corrosion. Concurrently, the rates of cathodic reactions, like the reaction involving oxygen, change in the opposite direction. This means that the cathodic reaction accelerates on the more noble metal and decelerates on the more active metal.
The accelerated corrosion of the more active metal, iron in this example, is known as galvanic corrosion. While the galvanic series provides information about the relative reactivity of metals, it does not indicate the extent to which corrosion rates will change. However, typically, galvanic corrosion poses a more significant issue when the metals involved are further apart in the galvanic series.
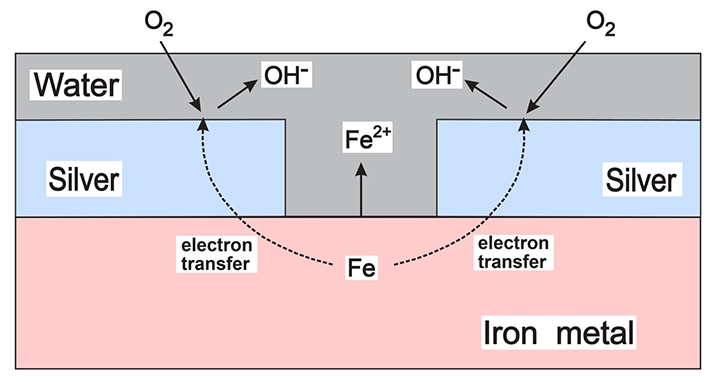
To illustrate these reactions, Figure above presents an example where a silver-plated layer is damaged, exposing the underlying iron. In this case, the corrosion is intensified due to the relative areas of the two metals. The larger surface area of the top silver layer provides ample space for oxygen to react, thus enhancing the corrosion process.
Examples of Galvanic Corrosion
Galvanic corrosion can occur in various scenarios where dissimilar metals or alloys are in contact with each other in the presence of an electrolyte. Here are a few examples of galvanic corrosion:
Aluminum and Stainless Steel
- When aluminum and stainless steel come into contact in the presence of an electrolyte, such as saltwater, galvanic corrosion can occur. The aluminum acts as the anode and corrodes more rapidly, while the stainless steel acts as the cathode and is protected. This can be observed in structures such as boat hulls or aluminum components attached to stainless steel fasteners.
Copper and Steel
- Galvanic corrosion can occur when copper and steel are in contact, especially in environments with high humidity or moisture. Copper acts as the cathode, remaining relatively protected, while the steel acts as the anode and corrodes more quickly. This can be seen in plumbing systems where copper pipes are connected to steel fittings.
Zinc and Steel
- In structures where zinc-coated (galvanized) steel comes into contact with bare steel, galvanic corrosion can take place. The zinc coating acts as the sacrificial anode, corroding to protect the underlying steel. This is commonly observed in galvanized steel structures, such as fences or roofing, where the zinc coating may degrade over time.
Magnesium and Aluminum
- When magnesium and aluminum alloys are in contact, galvanic corrosion can occur, particularly in the presence of an electrolyte like saltwater. Magnesium acts as the anode and corrodes preferentially, while aluminum acts as the cathode and is relatively protected. This can be seen in marine applications or in automotive parts where magnesium and aluminum components are in contact.
Carbon Steel and Cast Iron
- Galvanic corrosion can happen when carbon steel and cast iron are in contact, especially in the presence of moisture or corrosive environments. The carbon steel acts as the anode and corrodes more rapidly than the cast iron, which acts as the cathode. This can be observed in structures such as pipelines or machinery components.
Galvanic corrosion can occur between various pairs of metals. Here are a few more examples:
- Zinc as anode and copper as cathode: When zinc and copper are placed in an electrolyte, such as a water-based solution containing copper sulfate, galvanic corrosion occurs. Zinc acts as the anode and starts to oxidize, corroding gradually and forming zinc ions. Copper, acting as the cathode, gains electrons and is protected from corrosion. This process continues until the zinc electrode is completely dissolved. Hydrogen ions present in the electrolyte are also reduced to form hydrogen gas, observed as bubbles at the cathode.
- Steel and aluminum: When steel and aluminum come into contact in an electrolyte, galvanic corrosion occurs. Aluminum acts as the anode and undergoes corrosion, while steel is protected.
Prevention of Galvanic Corrosion:
To prevent galvanic corrosion, several methods can be employed:
- Avoid galvanic coupling: Do not place two dissimilar metals together that can undergo galvanic corrosion. Use metals that are electrochemically similar.
- Use insulating materials: If dissimilar metals must be in contact, insulating materials can be used between them to prevent the formation of a circuit and the flow of electrons.
- Paint: Applying paint to metal surfaces creates a barrier that covers the metal, preventing direct contact with the electrolyte and reducing the likelihood of galvanic corrosion.
- Avoid large cathode and small anode: Having a large cathode (protected metal) and a small anode (active metal) reduces the potential for dissimilar corrosion.
These examples demonstrate how the combination of dissimilar metals and an electrolyte can lead to galvanic corrosion, with one metal acting as the anode and corroding more rapidly while the other metal acts as the cathode and is protected. Proper material selection, insulation, or the use of protective coatings can help mitigate galvanic corrosion in such situations.








