Chloride Stress Corrosion Cracking (SCC) is a significant concern in various industries, particularly those involving the use of metals and alloys in corrosive environments.
This blog post aims to shed light on what Chloride SCC is, why it occurs, how to prevent it, and the importance of testing and inspection in detecting and mitigating its effects.
What is Chloride Stress Corrosion Cracking?
Chloride Stress Corrosion Cracking is a specific type of corrosion that arises from the combined action of tensile stress, specific alloy composition, and the presence of chlorides in the environment. It involves the formation of cracks in susceptible materials, leading to potential structural integrity issues and catastrophic failures.
“Stainless steels can develop cracks when subjected to both tensile stress and specific corrosive environments. This phenomenon is known as stress corrosion cracking (SCC).“
Among various environmental factors, the presence of chlorides is primarily responsible for causing SCC in stainless steels. While no stainless steel grade is completely immune to chloride-induced SCC, the level of resistance against this type of corrosion varies significantly among different stainless steel grades.
Mechanisms of Stress Corrosion Cracking
Stress corrosion cracking (SCC) occurs due to the combined action of tensile stress and a corrosive environment. There are several mechanisms involved in the initiation and propagation of SCC. Some common mechanisms are:
- Anodic Dissolution: In this mechanism, the corrosive environment leads to the dissolution of the metal at anodic sites, creating pits or localized areas of corrosion. The tensile stress then acts as a driving force for crack initiation and propagation from these susceptible sites.
- Hydrogen Embrittlement: SCC can be induced by the ingress of hydrogen into the metal lattice. The hydrogen atoms can cause embrittlement, reducing the material’s ductility and promoting crack formation under the influence of tensile stress.
- Film Breakdown: Protective oxide films or passive layers on the metal surface can break down in a corrosive environment, exposing the metal to direct attack. The combination of tensile stress and the aggressive environment can initiate cracks at these vulnerable sites.
- Environmentally Assisted Cracking (EAC): EAC occurs when the corrosive environment chemically assists the crack propagation process. This can involve processes such as corrosion-assisted slip, corrosion-assisted grain boundary decohesion, or stress-assisted corrosion.
- Transgranular and Intergranular Cracking: SCC can exhibit different crack paths within the material’s microstructure. Transgranular cracking occurs through the grains, while intergranular cracking follows grain boundaries. The susceptibility to either type of cracking depends on factors such as alloy composition, grain boundary structure, and environmental conditions.

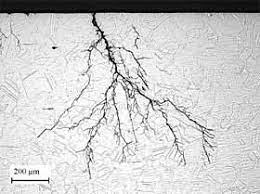
Chloride Stress Corrosion Cracking Crack appearance
Stress corrosion cracking (SCC) is a form of corrosion that occurs when a susceptible material is exposed to both tensile stress and a corrosive environment. Pitting can serve as a triggering factor for SCC, as the base of pits amplifies the stress.
Identifying SCC often requires the use of a microscope or other high-magnification devices. The cracks associated with SCC can exhibit either an intergranular or a transgranular morphology within the material’s microstructure.
The crack appearance of chloride stress corrosion cracking is characterized by branched transgranular cracks. Figure below illustrates an example of such cracking on a 6Mo super austenitic stainless steel (N08367) exposed to 0.2% chlorides at 500 °F (260 °C).
Why does Chloride Stress Corrosion Cracking occur?
Chloride SCC occurs due to a combination of factors:
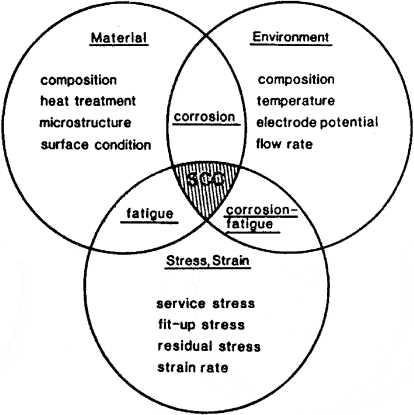
- Presence of Chlorides: Chloride ions have a high affinity for certain metal surfaces, leading to their accumulation. This accumulation creates an aggressive environment that promotes crack initiation and propagation.
- Tensile Stress: The presence of tensile stress, whether residual or applied, significantly increases the susceptibility to SCC. This stress can result from factors such as mechanical loading, welding, or thermal cycling.
- Alloy Sensitivity: Not all alloys are equally prone to Chloride SCC. Some alloys, such as austenitic stainless steels and certain nickel-based alloys, are particularly susceptible due to their microstructural characteristics.
Prevention of Chloride Stress Corrosion Cracking
Preventing Chloride SCC requires a comprehensive approach. Here are some preventive measures:
- Material Selection: Choose materials with improved resistance to Chloride SCC, such as duplex stainless steels or high-performance alloys, depending on the specific application requirements.
- Environment Modification: Minimize chloride concentration in the environment by implementing corrosion-resistant coatings, inhibitors, or desalination processes. Controlling temperature and pH levels can also be beneficial.
- Stress Reduction: Employ stress-relieving techniques, such as heat treatment or mechanical procedures, to minimize residual stresses and avoid excessive applied stresses.
- Design Considerations: Optimize designs to minimize stress concentrations, avoid sharp corners, and use appropriate welding techniques to reduce vulnerability to Chloride SCC.
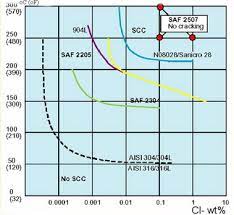
Chloride Stress Corrosion Cracking Testing
The resistance of stainless steel to chloride-induced stress corrosion cracking is commonly assessed using standardized boiling salt solutions. The table below presents the outcomes of testing conducted in boiling salt solutions of 26% NaCl (sodium chloride), 33% LiCl (lithium chloride), and 42% MgCl2 (magnesium chloride).
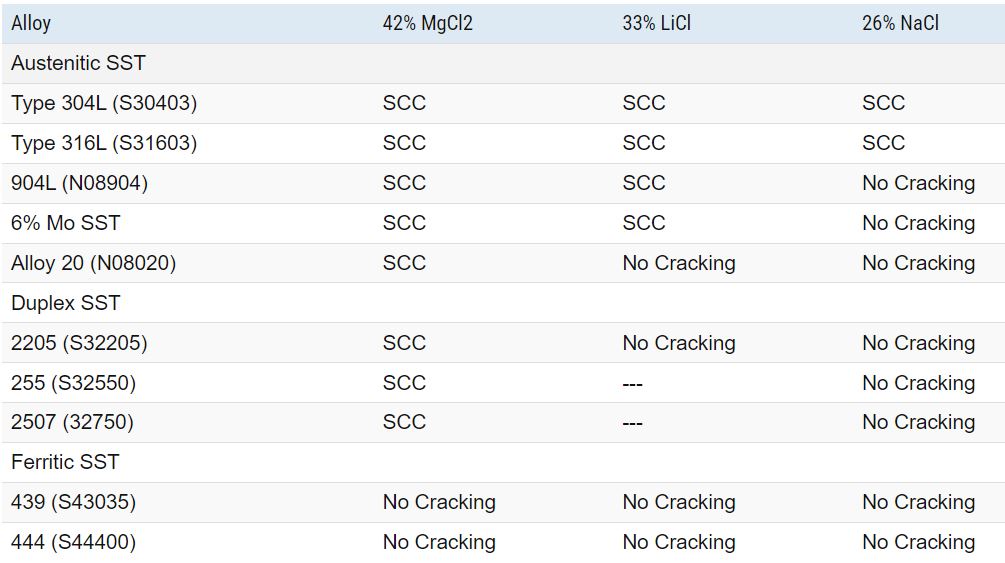
It’s important to note that the boiling LiCl and MgCl2 test solutions are highly aggressive compared to real-world applications. Only austenitic alloys with compositions similar to nickel-based alloys consistently demonstrate resistance to cracking in these rigorous test solutions.
| Salt Solution | Stainless Steel Performance |
|---|---|
| 26% NaCl | Varies among different stainless steel grades |
| 33% LiCl | Only austenitic alloys similar to nickel-based alloys resist cracking |
| 42% MgCl2 | Only austenitic alloys similar to nickel-based alloys resist cracking |
Materials Susceptible to Stress Corrosion Cracking
The susceptibility of stress corrosion cracking (SCC) can vary depending on the type of material used. Different materials have varying levels of susceptibility to SCC. Inadequate material selection can result in SCC when the chosen material is prone to this type of corrosion in the specific corrosive environment it is exposed to. Therefore, careful consideration of material properties and compatibility with the operating environment is crucial to prevent SCC.
Certain materials are more susceptible to stress corrosion cracking (SCC) compared to others. Here are some examples of materials that are commonly known to be susceptible to SCC:
- Austenitic Stainless Steels: While they offer excellent overall corrosion resistance, certain austenitic stainless steels, especially those with high nickel content, can be susceptible to SCC in chloride-containing environments.
- Aluminum Alloys: Certain aluminum alloys, such as those in the 2000 and 7000 series, are prone to SCC under specific conditions, including exposure to alkaline solutions, chloride ions, or high temperatures.
- Copper Alloys: Copper and its alloys, including brass and bronze, can be susceptible to SCC in the presence of ammonia, sulfur compounds, or other corrosive environments.
- Carbon Steels: Carbon steels, particularly those with higher carbon content, can be susceptible to SCC in environments containing hydrogen sulfide, ammonia, or acidic solutions.
- Titanium Alloys: Some titanium alloys, such as those containing alpha-beta phases, can exhibit SCC when exposed to certain chlorides, nitrates, or hot alkaline solutions.
Testing and Inspection of Chloride Stress Corrosion Cracking
Testing and inspection play a crucial role in identifying and managing the risks associated with Chloride SCC. Here are some common methods:
- Visual Inspection: Regular visual examinations can detect surface cracks and signs of corrosion. It is a cost-effective method for identifying visible damage.
- Non-Destructive Testing: Techniques like liquid penetrant inspection, magnetic particle inspection, ultrasonic testing, and radiographic testing can detect cracks and defects that are not visible to the naked eye, providing detailed information about size and location.
- Electrochemical Testing: Electrochemical methods such as polarization resistance and electrochemical noise analysis can assess the susceptibility of materials to Chloride SCC and monitor corrosion rates.
Conclusion
Chloride Stress Corrosion Cracking poses a significant risk to the integrity and reliability of metal structures in chloride-containing environments. Understanding its causes, implementing preventive measures, and conducting regular testing and inspection are crucial for mitigating the potential impacts of Chloride SCC.








